[ad_1]
November 17, 2023
4 min go through
The look for for home-temperature superconductors has endured scandalous setbacks, but physicists are optimistic about the field’s potential
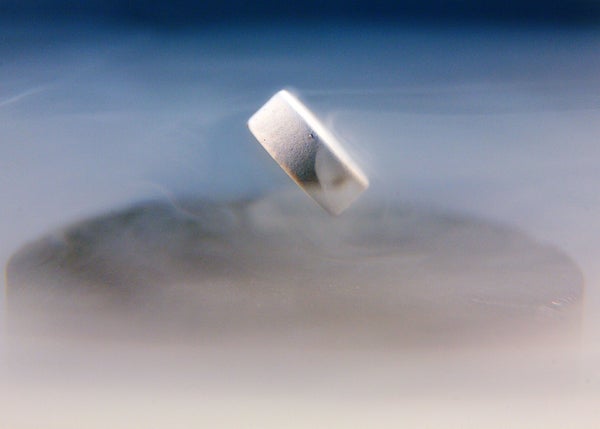
A magnet levitating over a nitrogen-cooled superconductor.
A Character retraction past week has put to relaxation the most recent declare of space-temperature superconductivity — in which scientists mentioned they had made a content that could perform electric power devoid of developing squander heat and with no refrigeration.
The retraction follows the downfall of an even extra brazen claim about a meant superconductor called LK-99, which went viral on social media previously this year.
Even with these large-profile setbacks, superconductivity scientists say the subject is taking pleasure in considerably of a renaissance (see ‘Timeline: Superconductivity milestones’). “It’s not a dying subject — on the contrary,” suggests Lilia Boeri, a physicist who specializes in computational predictions at the Sapienza College of Rome. The development is fuelled in aspect by the new abilities of pc simulations to predict the existence and properties of undiscovered supplies.
Substantially of the pleasure is concentrated on ‘super-hydrides’— hydrogen-abundant materials that have proven superconductivity at at any time-bigger temperatures, as very long as they are retained at superior force. The issue of the retracted Mother nature paper was purported to be this sort of a content, manufactured of hydrogen, lutetium and nitrogen. But do the job in the previous number of decades has unearthed several families of supplies that could have revolutionary attributes. “It truly does look like we’re on the hairy edge of getting able to discover a ton of new superconductors,” states Paul Canfield, a physicist at Iowa State College in Ames and Ames National Laboratory.
Surfing electrons
Superconductivity arises when electrons in a reliable mix to variety ‘Cooper pairs.’ This allows several extra electrons than usual to transfer in sync inside the substance, which in flip allows the electrons to carry currents with out generating waste heat.
In ‘conventional’ superconductors, electrons variety Cooper pairs when nudged alongside one another by vibrations in the content — mechanical waves that the Cooper pairs ride like surfers on a wave. Until finally the mid-2000s, researchers commonly imagined that this system would operate only at really lower temperatures, up to all over 40 kelvin. Superconductors designed of a one element all need temperatures lower than 10 kelvin to exhibit this residence. Magnesium diboride, a standard superconductor found in 2001 by a team led by Jun Akimitsu at Okayama College in Japan, elevated the history for the optimum temperature to 39 kelvin.
The basis for super-hydrides was laid out in 2004, when the late theoretical physicist Neil Ashcroft predicted that particular things would sort compounds with hydrogen that could superconduct at much better temperatures than could any other substance, if put under sufficient pressure to drive the hydrogen atoms closer collectively.
According to Ashcroft’s concept, the proximity of the hydrogen atoms would improve the frequency of mechanical vibrations, which would allow the content to get hotter when retaining its superconductivity. But there was a capture: to even exist, some of these supplies would involve pressures comparable to people in Earth’s main.
Improvements in carrying out significant-force experiments on very small samples inside of a diamond anvil — and measuring their outcomes — led to a breakthrough in 2015, when physicist Mikhail Eremets at the Max Planck Institute for Chemistry in Mainz, Germany, and his collaborators initially shown superconductivity in a tremendous-hydride, hydrogen sulfide. Considering that then, researchers have predicted the existence of quite a few other superconducting supplies in this family — some of which have been identified, such as calcium-based cage-like structures identified as clathrates.
At current, the ‘hottest’ superconductor of any kind is viewed as to be lanthanum decahydride, a member of the super-hydride class that is verified to be a large-stress, common superconductor at temperatures of up to at the very least 250 kelvin.
Innovative simulations
Eremets and many others say that the interaction of idea, simulation, supplies synthesis and experiment has been vital to development. Beginning in the early 2000s, it became feasible for simulations to predict no matter whether a substance with a specific crystal composition and chemical composition could be a superconductor, and at what temperatures it could show this residence. But the subsequent big shift was the introduction of algorithms later that ten years that could forecast not just the attributes of a product, but what components can sort from a given combine of things. “Until then, a vital bit was missing: knowing whether or not a compound can sort in the 1st spot,” says Boeri.
The discovery in 2015 that hydrogen sulfide is a superconductor was constant with laptop or computer simulations carried out the 12 months before. Without fast innovations in framework prediction, the discovery of hydrogen-abundant superconductors “probably would have not took place for one more century,” says Artem Oganov, a components scientist at the Skolkovo Institute of Science and Technologies in Moscow, who has pioneered composition-prediction algorithms. His ‘evolutionary’ algorithms, in individual, locate the configuration of atoms with the most affordable strength — and for that reason ideal chance to sort and keep on being stable — at a specified tension.
Simulations are particularly vital for predicting the conduct of products at higher pressures, under which atoms are pushed so close to a person yet another that they start off to interact not only through their outer electrons, but also with additional internal types, throwing chemistry-textbook dogma out of the window. An illustration of this is lithium hexahydride, which can exist only at large pressures. “Anybody in standard-chemistry class would notify you that one thing like LiH6 cannot be steady,” claims Eva Zurek, a computational chemist at the College at Buffalo in New York.
This article is reproduced with permission and was initial released on November 16, 2023.
[ad_2]
Source connection