[ad_1]
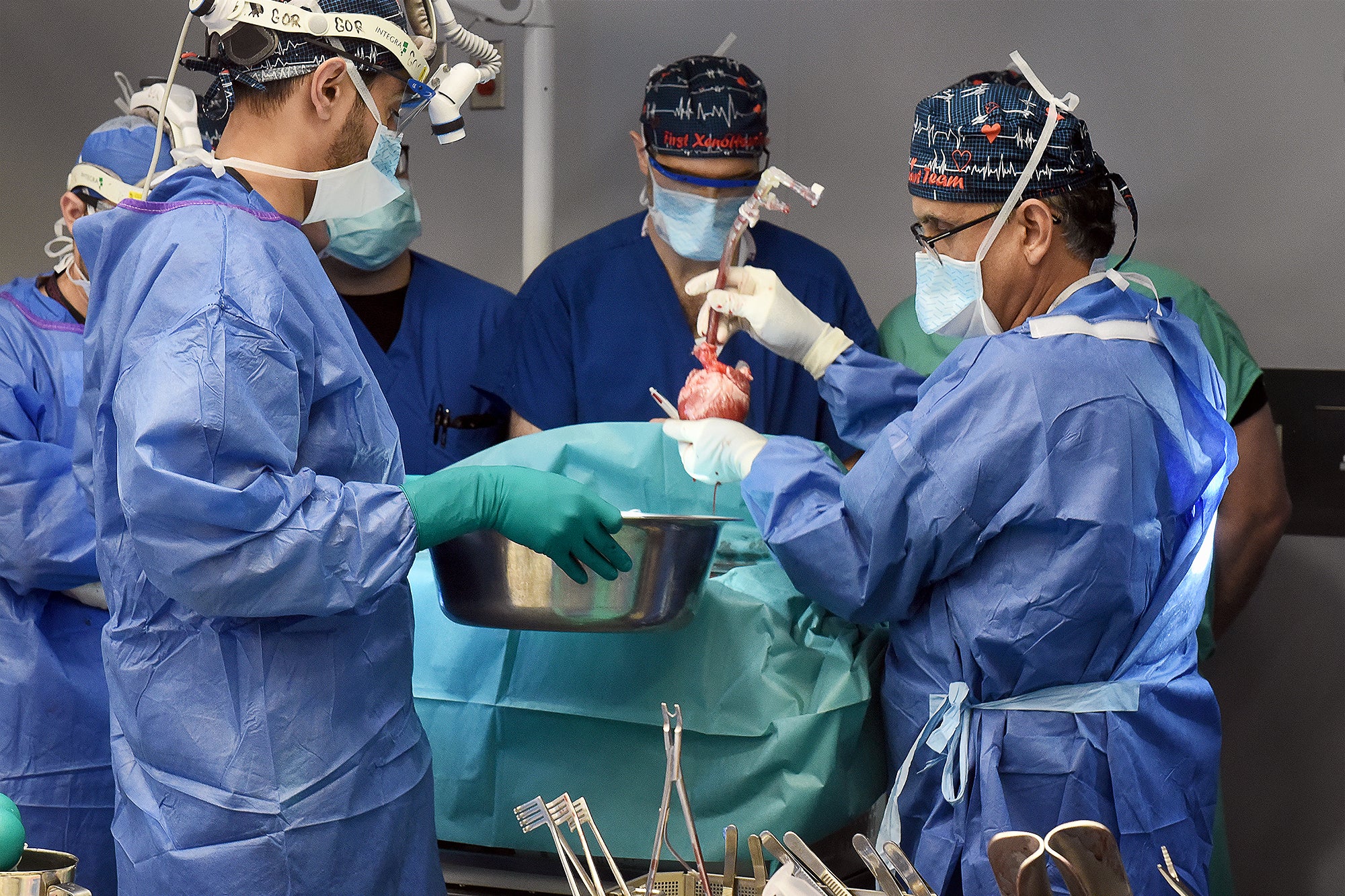
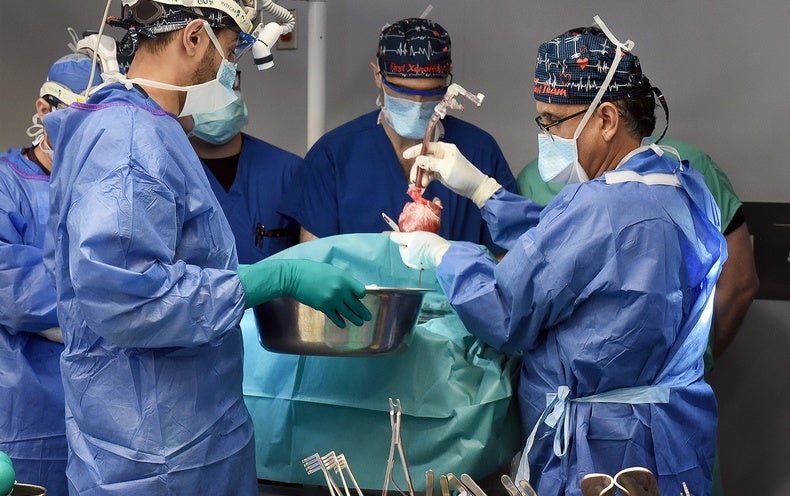
Late last thirty day period a group of scientists at the University of Maryland College of Medicine transplanted a genetically modified pig coronary heart into a person—the next these operation at any time attempted—and it has stored him alive for the previous handful of months. The affected person, 58-year-previous Lawrence Faucette, underwent the hugely experimental treatment under a “compassionate use” pathway, in which the U.S. Foodstuff and Drug Administration permits an unapproved remedy when a particular person is severely sick or dying and has no other alternatives out there. Faucette was not qualified for a regular human heart transplant due to the fact he had peripheral vascular illness and other problems, which narrowed the outlook for results.
As of this 7 days, Faucette is continuing to get well and doing actual physical treatment. “He’s had a tough time,” nevertheless, states Bartley Griffith, a surgeon at the University of Maryland, who carried out Faucette’s procedure as well as the past one. According to Griffith, Faucette was residing at house when the Fda to start with accredited the surgical treatment, but he was subsequently hospitalized with fluid in his lungs. Then he endured a cardiac arrest the night time before the surgical procedures. Nonetheless, he has so significantly responded nicely to the transplant—and was sitting up in a chair two times afterward. “The following benchmark will be acquiring him up and strolling,” Griffith states.
More than 100,000 people today are waiting for an organ transplant—most of them for kidneys—so scientists have very long been exploring xenotransplantation: transplanting other species’ organs into humans. To stop the human immune system from attacking these alien organs, experts have begun to breed genetically modified donor pigs that deficiency certain genes or have other genes additional.
In the past pair of years, pig xenotransplants have been analyzed in equally nonhuman primates and deceased humans—but the best target is to perform human scientific trials on a more substantial scale. The outcomes of the recent compassionate use transplant will likely affect the FDA’s thing to consider of whether or not and when to allow for these types of trials to acquire position. Several scientists hope this could transpire in the future year or two.
“I would love to see heart [xenotransplantation in] a clinical demo following calendar year and kidney [xenotransplantation trials] soon thereafter,” states Jayme Locke, director of the division of transplantation at the University of Alabama at Birmingham, who was not concerned in the hottest experimental operation. Locke and her colleagues have performed a number of kidney xenotransplants in human beings who had experienced brain demise. “The Food and drug administration holds those playing cards, and I imagine it is going to really count on what their chance tolerance threshold is,” she states. “But I’m hopeful. I feel the Fda would like to see this transpire.”
In January 2022 Griffith and his team at the College of Maryland transplanted a genetically modified pig coronary heart from the corporation Revivicor into a individual, David Bennett, Sr., who lived for two months before the coronary heart unsuccessful, and he handed absent. The heart was later on discovered to be infected with a pig virus that had escaped screening, though other elements might have also played a position in the transplant’s failure and Bennett’s loss of life.
“We took a rather good swing at the ball the very first time, and we bought really near to a extended achievements, we feel,” Griffith says. There had been some unforeseen situations in that initially xenotransplant that may possibly have affected its end result, these as the pig virus that was later located in the heart, Griffith adds. Considering the fact that then his staff and many others have designed much better procedures to exam for these viruses.
Bennett’s relatives is happy he was ready to choose portion. “He lived for two months, and we received to invest much more time with him. So I was grateful for that,” suggests his son, David Bennett, Jr., who hopes Faucette’s surgery will be successful. “I’m thankful for each aspiration and hope and each man or woman that is associated in this and the capability that it has to go forward.”
1 important variance among the initial and 2nd surgical procedures is that despite the fact that Faucette was thought of terminally ill, he was significantly much healthier than Bennett was at the time of his treatment. Compared with Bennett, Faucette had been residing at residence till soon prior to the transplant and was considerably extra mobile, according to Muhammad Mohiuddin, director of the Cardiac Xenotransplantation System at the College of Maryland Faculty of Medication, who is controlling Faucette’s anti-transplant-rejection routine.
Other researchers concur that Faucette was a more proper prospect for such a novel process. “My total emotion is that this patient was in substantially much better form than the prior patient,” suggests Nader Moazami, a cardiothoracic transplant surgeon at NYU Langone Health and fitness, who was not included in Faucette’s transplant. “Part of the challenge when we have a affected person who is extremely, incredibly sick—and you go into carrying out experimental xenotransplantation, wherever we nevertheless really don’t always know exactly what combination of immunosuppressive brokers is good—is that those people clients are pretty prone to building a wide range of difficulties.” Past year Moazami and his colleagues transplanted genetically modified pig hearts into two folks who experienced endured mind loss of life, and the organs functioned nicely for several times.
Both of those Bennett’s and Faucette’s procedures applied common immunosuppressive medicines in addition to an experimental one particular. With Bennett, the workforce employed an experimental antibody drug called KPL-404, which blocks a receptor termed CD40 that activates the host’s B cell and T mobile immune responses, which can guide to the rejection of a international organ. With Faucette, the staff employed a drug named tegoprubart, which was made by Eledon Pharmaceuticals and blocks the molecule, or ligand, which binds to CD40. Tegoprubart has been analyzed in period 2 medical trials for human kidney transplants but is not still Fda-accredited.
The team is also operating with worldwide laboratories that are applying artificial intelligence to evaluate biopsies of Faucette’s coronary heart tissue, an assessment that Griffith states could detect early signs of tissue rejection.
The Food and drug administration will be carefully observing the development of Faucette’s restoration, which could inform the agency’s final decision to approve medical trials of xenotransplantation. Locke thinks the initial trials will very likely require hearts, not kidneys, mainly because dialysis can keep persons with kidney sickness alive for a number of a long time. There is no comparable substitute for heart function. Dialysis is nonetheless an imperfect option, however, and Locke hopes kidney xenotransplants will be subsequent. “I believe there is a frequent misperception that dialysis is an ideal choice, and it is not,” Locke says. “People might are living a bit lengthier on dialysis” than on existence-extending heart therapies, she says, but dialysis just cannot switch kidney functionality in the very long phrase. Only transplants can do that. “We now have an substitute organ supply that I truly consider is greater than dialysis,” she claims. “And it is time to be equipped to take a look at that.”
[ad_2]
Resource connection