[ad_1]
CRISPR, the gene-modifying technological know-how that has revolutionized biological study, is at last out there as a clinical treatment method with regulatory acceptance. On December 8 the U.S. Foodstuff and Drug Administration approved the 1st CRISPR remedy for sickle cell illness.
The treatment, termed exa-cel and designed by the firms Vertex and CRISPR Therapeutics, edits a gene associated in red blood cell shape and function. It seems to functionally remedy the ailment for at the very least 1 12 months. The FDA’s decision would make the U.S. the 2nd nation to approve a CRISPR remedy, pursuing exa-cel’s acceptance for sickle mobile sickness in the U.K. in November.
Scientifically talking, exa-cel is “an incredible asset to have,” claims Michael DeBaun, a hematologist at Vanderbilt College. But it is continue to early to say regardless of whether the remedy will be long-lasting and without the need of side effects, he provides.
The Food and drug administration also approved an additional style of gene therapy for sickle mobile disease today called lovo-cel, which is built by the firm bluebird bio.
What is sickle cell disorder, and how does the new CRISPR therapy get the job done?
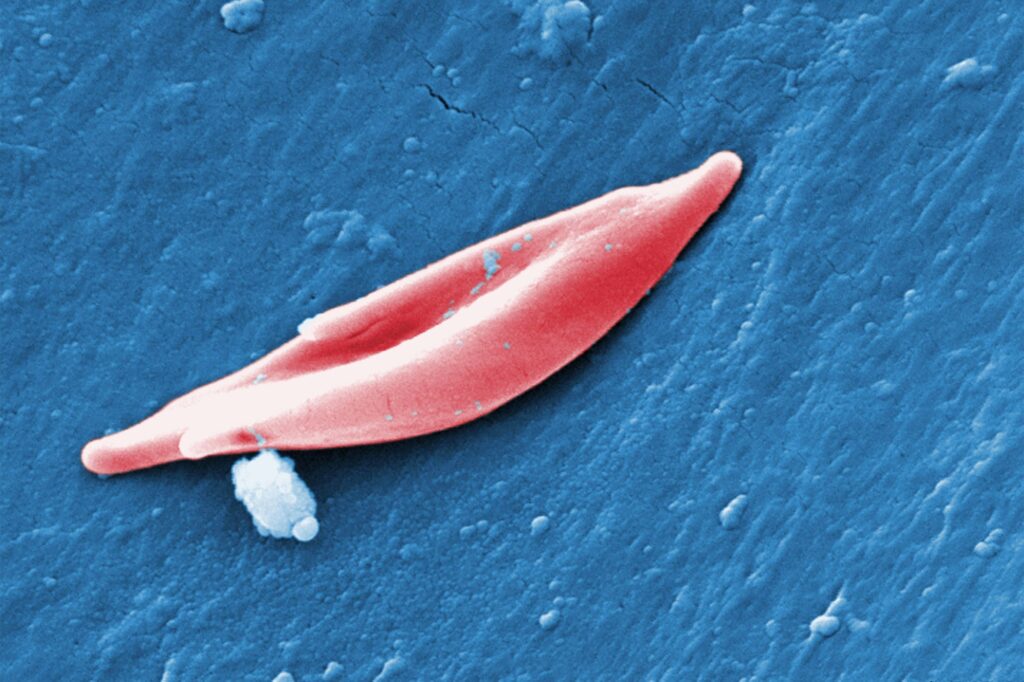
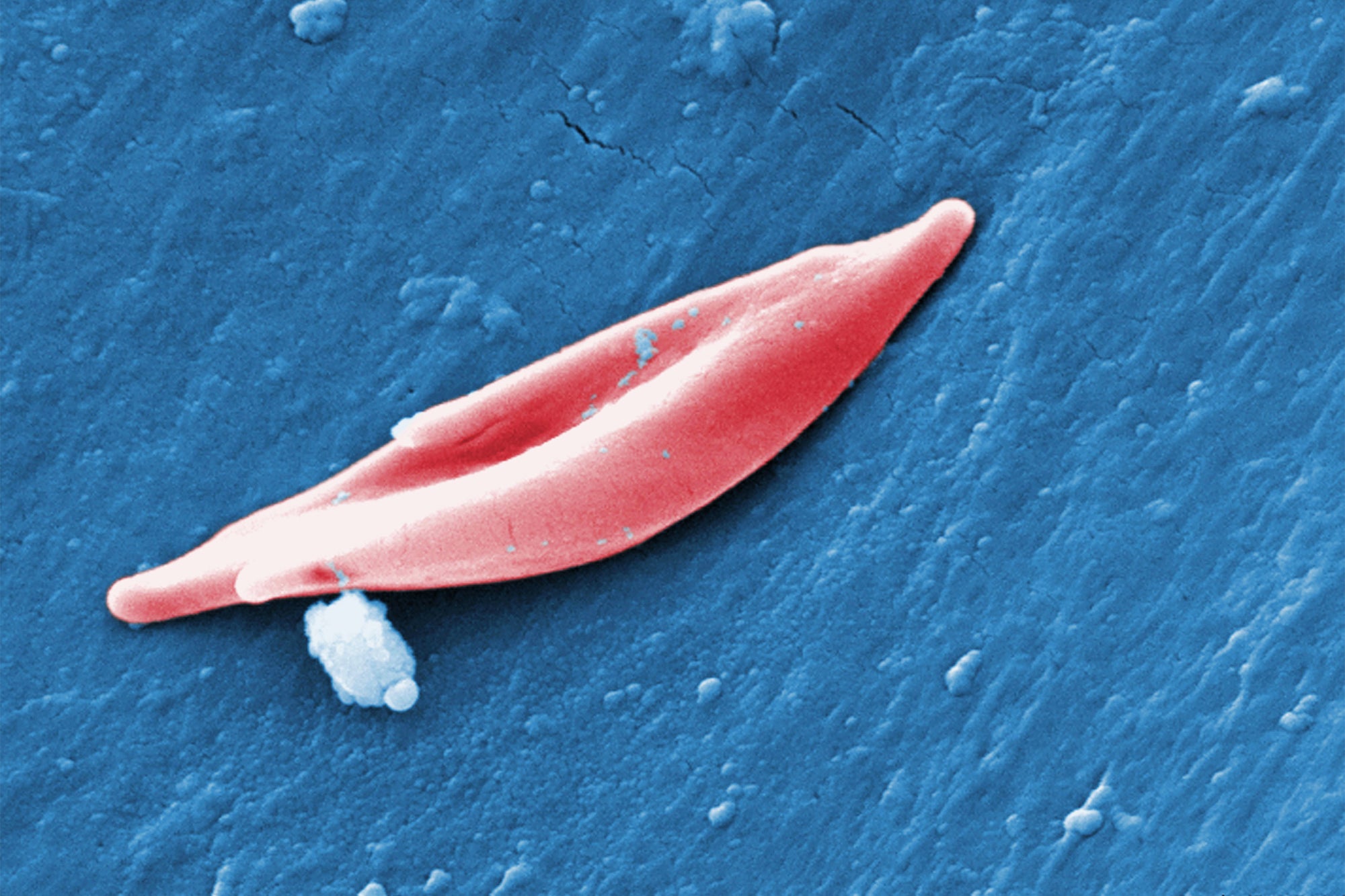
The CRISPR technique utilised in exa-cel targets genes that generate hemoglobin, the protein that carries oxygen in blood cells. In a variety of sickle mobile disease called sickle cell anemia, mutations in a gene identified as HBB affect the protein’s framework, creating it to twist ordinarily round crimson blood cells into a curved sickle condition. These sickled cells clog blood vessels, main to extreme pain and exhaustion. In a relevant problem called beta-thalassemia, which often happens with sickle cell anemia, the overall body does not deliver more than enough hemoglobin or red blood cells, resulting in symptoms brought on by small blood oxygen concentrations. These indications consist of exhaustion and progress issues in kids.
Exa-cel directs an enzyme called Cas9 to a gene, termed BCL11A, that generally stops the body from making a kind of hemoglobin located only in fetuses. Cas9 deactivates BCL11A in bone marrow stem cells, exactly where red blood cells are built, by reducing its DNA, and the cells start generating the fetal hemoglobin and generating crimson blood cells with a ordinary spherical shape. In the new therapy, medical professionals take out a person’s individual bone marrow stem cells, edit them with exa-cel, demolish the rest of the person’s untreated bone marrow and then reinfuse the edited cells.
Since these edited cells sooner or later repopulate the physique, exa-cel is thought of a “curative” treatment that will theoretically very last the relaxation of the recipient’s lifetime, while Vertex and CRISPR Therapeutics have adopted most of their demo contributors for considerably less than two decades.
How powerful is the therapy?
So much exa-cel has only been examined in all around 100 men and women with either sickle cell anemia or beta-thalassemia. Yet, in 2019 the Fda gave the corporations a “fast-track” acceptance that permits it to exam the treatment in smaller sized teams of individuals than would ordinarily be expected.
In these trials, which are nevertheless ongoing, 29 of the 30 analyze contributors with sickle mobile anemia had no ache for a person yr in the 18 months next their exa-cel transfusions. And 39 out of 42 beta-thalassemia patients no extended essential blood or bone marrow transplants—the conventional therapy for this disease—for a person year after the exa-cel intervention. Vertex and CRISPR Therapeutics are continuing to monitor the remaining individuals who have not still arrived at this time point and will abide by all individuals for up to 15 decades.
What are the challenges?
The outcomes submitted to the Food and drug administration suggest that exa-cel has no major adverse well being impacts, though it can cause aspect results this kind of as nausea and fever. But DeBaun details out that the contributors have only been tracked for a limited time and that troubles could crop up afterwards.
Another concern lifted by the Fda is that the Cas9 enzyme could remain active and slash the genome in spots other than BCL11A, developing so-called off-goal mutations. The corporations modeled the most most likely sites in the genome where by the enzyme could cut and discovered no evidence that this had transpired in trial members. “In mild of any new therapy, we continue being cautiously optimistic,” DeBaun suggests.
Besides exa-cel, what are some other promising therapies for sickle cell condition?
Bluebird bio’s lovo-cel, the other gene treatment that was permitted now by the Food and drug administration, works by using a viral vector to provide a practical variation of an adult hemoglobin-creating gene and insert it permanently into a person’s genome. The facts bluebird bio submitted to the Fda showed lovo-cel was effective in 36 men and women who ended up adopted for a median of 32 months. Dozens of scientific studies are investigating other styles of gene therapies for sickle mobile anemia and beta-thalassemia that provide normal versions of HBB or other genes to the body.
Researchers have found that a procedure identified as haploidentical transplant could also overcome sickle cell anemia. In this treatment, which is now widely used to handle specified cancers, a person’s bone marrow cells are changed with individuals from a father or mother or sibling who is 50 p.c genetically similar to the recipient but does not have the sickness. Benefits that will be offered up coming week at the American Culture for Hematology’s yearly conference identified that 88 per cent of grown ups who been given these transplants ongoing to make ordinary red blood cells following two many years. DeBaun claims this approach could be specifically practical in small- and middle-profits countries since it is very likely to price significantly considerably less than gene modifying or gene treatment.
Will exa-cel or lovo-mobile be obtainable to absolutely everyone with sickle mobile condition?
Like most gene editing therapies, exa-cel and lovo-mobile are probably to be quite highly-priced. Neither Vertex, CRISPR Therapeutics nor bluebird bio have explained how a lot their respective therapies will charge, but estimates propose that the price for each and every could be as much as $2 million for every individual. It is however unclear no matter whether insurers, primarily governing administration solutions this sort of as Medicaid, will go over the therapies or in which scenarios they will do so. Sickle mobile disorder disproportionately influences people of African descent, together with African Americans, who are much more possible to have community coverage via Medicaid than most other teams in the U.S.
DeBaun suggests that selecting whether to pursue CRISPR gene enhancing or yet another solution these kinds of as haploidentical transplant will require to be a shared selection by clients, their families and their medical professionals. Even if gene modifying proves to be far more effective at completely curing the sickness, it is probable to be much less greatly out there and may acquire lengthier than a transplant from a donor.
Nonetheless, DeBaun suggests that exa-cel is a great to start with step, and he expects that the know-how will increase as far more is acquired about CRISPR therapies above the up coming ten years. “This is the first mile of a marathon,” he says.
[ad_2]
Source hyperlink