[ad_1]
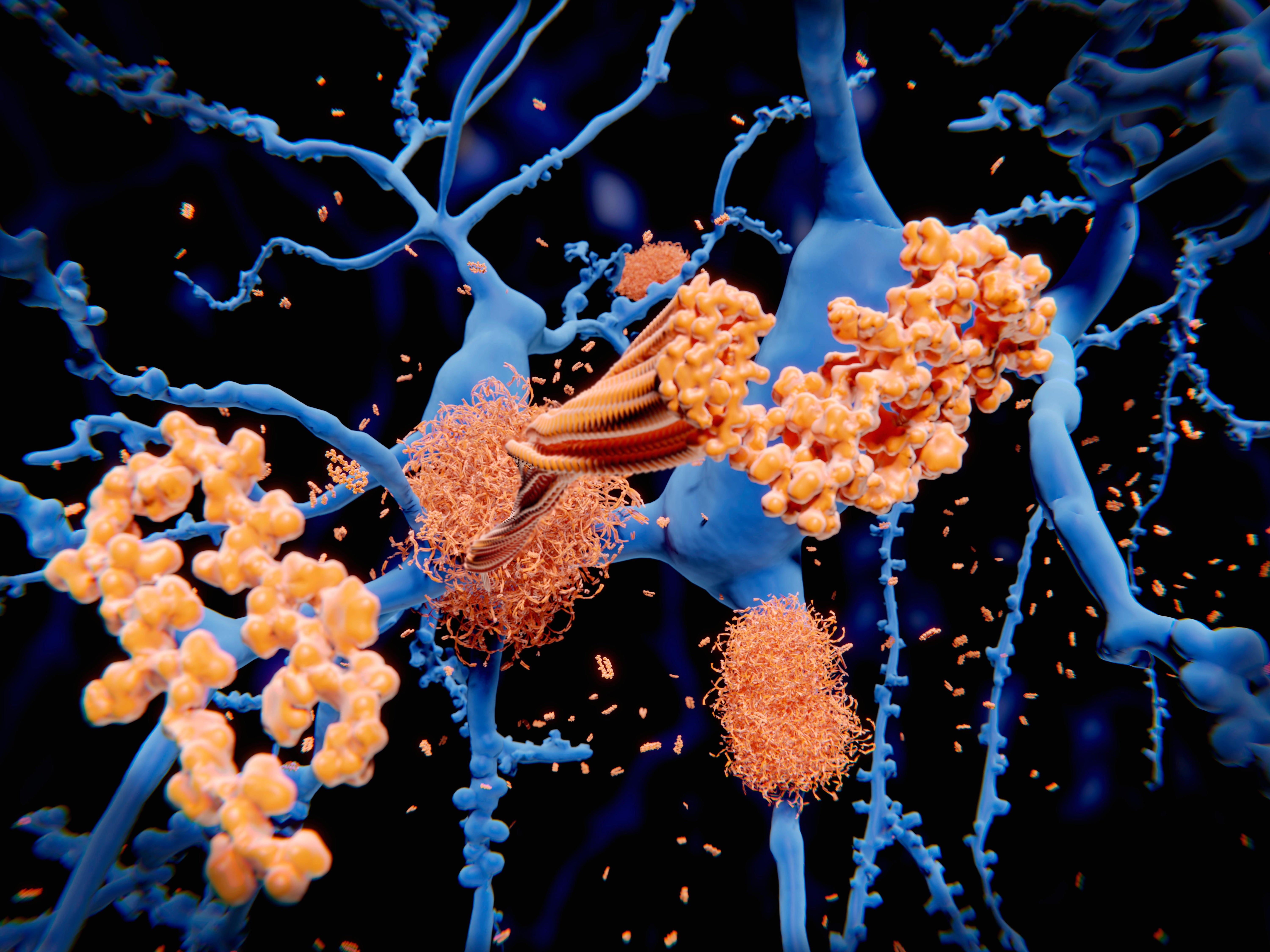
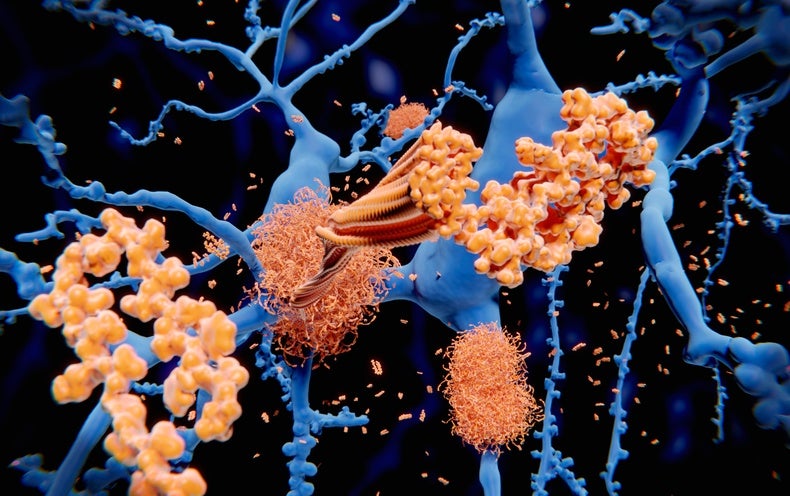
For the next time, an experimental drug has been revealed to cut down the cognitive decrease connected with Alzheimer’s sickness. On 3 Might, pharmaceutical company Eli Lilly announced in a push launch that its monoclonal antibody donanemab slowed mental decline by 35% for some participants in a 1,736-human being trial — a amount comparable to that for competitor drug lecanemab. But researchers alert that right up until the full results are revealed, thoughts continue to be as to the drug’s clinical usefulness, as properly as whether the modest benefit outweighs the chance of unsafe aspect outcomes.
Like lecanemab, donanemab targets amyloid protein, which is considered to result in dementia by accumulating in the mind and damaging neurons. The demo final results provide solid proof that amyloid is a important driver of Alzheimer’s, says Jeffrey Cummings, a neuroscientist at the University of Nevada, Las Vegas. “These are transformative in an enormously significant way from a scientific point of see,” he adds. “They’re great.”
But Marsel Mesulam, a neurologist at Northwestern College in Chicago, is extra careful. “The results that are explained are incredibly significant and amazing, but clinically their significance is doubtful,” he states, incorporating that the modest outcome implies that things other than amyloid add to Alzheimer’s disease progression. “We’re heading to a new era — there’s area to cheer, but it is an era that must make us all incredibly sober, knowing that there will be no single magic bullet.”
In the push release, Eli Lilly said that folks with mild Alzheimer’s who gained donanemab confirmed 35% a lot less clinical decrease above 18 months than did these who been given a placebo, and 40% a lot less decrease in their ability to perform day-to-day responsibilities. The enterprise suggests that it will current the total final results at a conference in July and publish them in a peer-reviewed journal. It plans to utilize for approval by the US Food stuff and Drug Administration (Fda) in the subsequent two months.
Promising therapies
Food and drug administration approval would make donanemab the third new Alzheimer’s treatment method in two a long time. In January, the company granted accelerated acceptance to lecanemab, designed by Biogen in Cambridge, Massachusetts, and Eisai in Tokyo. A study1 published in November showed that lecanemab slowed cognitive decline in 1,800 people by 27% over 18 months. The Food and drug administration experienced previously permitted aducanumab, also created by Biogen and Eisai, on the foundation of proof that it could lessen amyloid plaques in the mind, although it is nevertheless unclear irrespective of whether this sales opportunities to a meaningful scientific benefit for men and women with the sickness.
Eli Lilly’s donanemab trial differed from Biogen’s lecanemab a single in that persons stopped having the drug after their amyloid stages experienced dropped under a selected threshold. “The rationale is, if the target is gone, why keep taking pictures?” Cummings claims. In accordance to the push release, about 50 % of the demo participants were able to quit using the drug in a lot less than one yr.
Diana Zuckerman, president of the Countrywide Heart for Well being Research, a non-income feel tank in Washington DC, concerns that halting the drug could lead to the disorder to rebound or worsen, as is the situation with many psychiatric medicine. She warns that for a longer time-phrase stick to-up scientific tests will be necessary. “Any time you’re doing nearly anything that impacts the mind, you truly do have to be cautious,” she suggests.
Eli Lilly also identified that donanemab worked ideal in persons whose brains contained only reasonable amounts of a different protein, referred to as tau, that is also associated with Alzheimer’s progression. The organization experienced calculated its effects amongst its 1,182 trial individuals who had moderate tau concentrations, but reported that the enhancement was however statistically important when they mixed these sufferers with the 552 who had higher levels of tau.
Brent Forester, a geriatric psychiatrist at McLean Hospital in Belmont, Massachusetts, claims it is “fascinating” that eliminating amyloid also impacts tau: the romance amongst the two proteins, and their respective roles in sickness development are not thoroughly comprehended. “If we could fully grasp that better, we may comprehend why taking away amyloid could have a scientific result,” he says.
Bleeding and seizures
Like lecanemab, donanemab carries a substantial threat of aspect outcomes — especially a set of disorders termed amyloid-associated imaging abnormalities (ARIA) that can lead to seizures and bleeding in the mind. Scientists imagine that by attacking amyloid plaques, the antibodies inadvertently weaken blood vessels in the brain, and the consequences are particularly pronounced between men and women who are having anticoagulant medication. Eli Lilly’s press release explained that ARIA rates had been many situations greater in people who obtained donanemab than in all those who obtained placebos, and a few individuals in the demo died following going through the ailment.
“The side outcome is the largest worry for all of us right now,” claims Forester, who led earlier trials of donanemab and is currently performing on a lecanemab demo. He adds that individuals with moderate cognitive impairment purpose relatively properly, and that even 3 deaths may possibly be plenty of to sign that the possibility of facet consequences outweighs the profit of taking the drug.
Thoughts also remain about information and facts that is lacking from the announcement, which include regardless of whether donanemab worked at all between people today who experienced superior ranges of tau. “This total publication-by-press-launch is genuinely poor,” Zuckerman says.
Moreover, the results that Eli Lilly launched show only a slowing of cognitive decrease relative to the placebo team, alternatively than how much donanemab affects the absolute amount of a person’s decline. It’s unclear, Zuckerman claims, irrespective of whether that difference is fantastic sufficient to be apparent to individuals with Alzheimer’s and their people.
With at minimum a few monoclonal antibodies shortly to be on the market place, Mesulam worries that pleasure about them will lower drug companies’ enthusiasm for acquiring medicine for Alzheimer’s targets other than amyloid. “The following 20 to 25 decades will be taken up by greater amyloid medication,” he says. The Alzheimer’s current market is probable to be quite worthwhile for drug companies — lecanemab, for occasion, fees a lot more than US$26,000 for every 12 months of remedy — but Mesulam anxieties that the price of Alzheimer’s medicines will pressure the US health and fitness-treatment procedure.
Continue to, the first effects offer “further assist that this treatment will have some part with the proper individuals at the right time in illness”, Forester claims. “I’m cautiously optimistic.”
This posting is reproduced with permission and was first posted on Could 4, 2023.
[ad_2]
Resource backlink